If you are in the pharmaceutical, chemical, food, cosmetic and allied industries, you need to keep yourself abreast with the latest techniques and methods of handling and processing your material in an efficient and cost-effective manner.
We can provide you with a wealth of professional knowledge and wisdom of the process industry that will help you solve your process problems.
Click through the following articles to learn more:
IBC Loading using Vacuum Transfer
Vacuum transfer into an IBC offers a reliable dust-tight transfer that is highly flexible and easily adapted to various process changes. Using vacuum transfer is an ideal material handling solution where there is restricted space above the IBC. Even in tight spaces, loading rates of 1,000 kg per hour are often possible.

"Vacuum transfer is a simple, fully-contained method for loading an IBC"
To ensure the best possible powder transfer from the vacuum transfer hopper into the IBC, a dust-tight seal is essential. This can take the form of a dust cap, flexible silicone sleeve, inflatable sealing system or a split butterfly docking system, to give the highest level of integrity. It is worth remembering that it is essential to vent the IBC or receiving container, in order r to disperse displaced air during filling. This is easily done using a vent filter. Vent filters can be simple fabric sock-type filters or miniature HEPA filters.
Material pick-up can be achieved in a variety of ways. The most common method is of material pick-up is using a hand held vacuum wand. To minimise any dust concerns during this process a localised dust extract system can be used. Alternatively a Sack-Tip Station or feed bin provides a semi-automated method of material pick-up. These systems can incorporate a dust-hood and integrated or external dust extraction to further help improve containment. Finally, powder can be drawn straight from the outlet of one IBC and transferred to another.
One major advantage is that other processes such as sieving or milling can be performed 'in-line'. This helps reduce operator exposure and eliminates the need for a separate process step providing a major cost benefit. From pick-up point to discharge a fully contained system helps protect both the environment and personnel from the hazards of airborne dust particles.
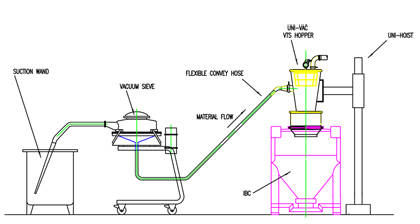
Using vacuum transfer, processes such as milling and sieving can be performed ‘in-line’
Improved containment using vacuum transfer for IBC loading also means reduced waste which further contributes to overall cost savings. Taking into consideration the benefits of flexibility, containment, labour savings and ease of cleaning, vacuum transfer for the loading of IBC’s and other bulk containers is the normally the optimal solution. For more information on Hanningfield’s vacuum transfer systems, please click here, or click HERE.
5 Tips for Optimising your Powder Transfer System
Process Tips: 5 Tips for Optimising your Powder Transfer System
There are various means to ensure optimum performance of your powder transfer system. Below are some of the simple, yet essential checks you can undertake:
1. It is important that the filter cleaning system used during powder transfer in operating efficiently. Filters are at the heart of any powder transfer system and should be checked and cleaned regularly. Blocked filters can constrict the airflow and can significantly hamper throughput as a consequence. Cartridge-type filters can be cleaned using a vacuum cleaner. Fabric-type filter bags can be washed.
2. Seals and gaskets must be in perfect condition and fitted properly to avoid air-leaks. Check seals and gaskets and remove any residual product that may cause air leaks. Similarly to above, any problems here can cause a significant drop in performance as the air flow suffers.
3. Air entrainment is vital to the correct operation of a lean-phase vacuum conveying powder transfer system. It is important that adequate air-bleed systems are incorporated into and convey line and checked for correct operation allowing air to be introduced when needed. Hanningfield offers a range of air-entrainment devices to ensure peak performance is achieved.
4. Good 'earthing' of the system in essential to avoid static build up. Ensure all flexible hoses are embedded-wire type and correctly earthed at each end. Confirmation of a continuous 'earth' throughout the system should be checked periodically using a multi-meter.
5. Finally, all vacuum conveying powder transfer systems rely on a timed sequence of operations, such as convey duration, discharge durations and length of filter clean cycle etc. It is important that these settings are correct to provide optimum performance. Control sequences should be regularly checked.
If a system is not correctly maintained, performance levels can fall significantly. For process-specific recommendations and best-practice, please just ask a question to our team of experts by clicking HERE.
Tablet Reclaiming
Reclaiming Tablets
Some manufactured tablets may be off-specification. However, even when a tablet fails final inspection, all may not be lost. Rather than discard the tablets, they can be reclaimed by milling the tablet back down to its powdered form. The product can then be re-worked for process re-introduction, significantly helping reduce waste and increase productivity.

Off-spec tablets can be safely milled for process re-introduction
Typical causes of off-specification tablets are; over-weight or under-weight, poor appearance, incorrect hardness and incorrect packaging. In fact, any condition which threatens to scrap a batch of valuable finished goods can be saved by using a conical screen mill to overcome the problem.
The hygienic and highly flexible design of the Uni-Mill is ideal for sanitary applications and a fully validated mill is the perfect way to ensure product integrity is maintained. It is possible to employ the same machine used for size reduction of pre-compression material as for the reclaim process. This provides a very cost effective solution for both the production of new material as well as re-worked material all performed on one machine.

Cone mills are a hygienic method of size reduction
A wide range of interchangeable tooling can be supplied with the conical screen mill and special tooling can be used for particularly hard tablets. Tooling is also available to mill filled capsules. Hard Shell capsules can be loaded into the mill in the normal way and the capsules are then shredded and torn away from the contents. The resulting mix of broken capsules and powders can then be sieved to ensure the final powder is fully separated and can be safely re-used as required.
As production costs continue to rise, the case for reclaiming off-specification products gets increasingly stronger. Hanningfield is able to provide appropriate cost-effective solutions to make this process a viable and practical option.
Improving tablet production and handling with pneumatic conveying
Improving tablet production and handling with pneumatic conveying
Article from Healthcare Packaging Online
Vacuum technologies can streamline production, reduce costs, and provide sustainability benefits.
Compressed tablets are still the most popular dosage form for pharmaceuticals. More than 75% of pharmaceutical products are sold in solid dosage form. Strong growth is predicted for compressed tablets due to the explosion of the nutraceuticals market, especially in the United States.
The potential for growth in tablet manufacturing is high. However, several challenges and trends are threatening the profit potential. Today, pharmaceutical manufacturers are challenged to evolve their production processes in order to survive and excel in an increasingly competitive industry. While product quality has always been of paramount importance, strenuous economic times and continually inflating drug prices are heightening consumer demand for lower-priced prescriptions. Tablet manufacturers are more than ever seeking new ways to automate their lines to speed and streamline production.
By automating the conveying process, vacuum technologies can improve productivity and enhance quality. In addition, selecting a vacuum technology that is maintenance-free and energy efficient can allow tablet manufacturers to cut costs and meet challenging consumer demands for quality pharmaceuticals and lower prices.
Offering a safer environment
In general, reducing manual labor through automated vacuum conveying can improve working conditions by reducing heavy lifting. In addition, American manufacturers must abide by Safety, Health and Environment (SHE) regulations enforced by the U.S. Dept. of Occupational Safety & Health Administration. In Europe, the European Foundation for the Improvement of Living and Working conditions (Eurofounda) oversees the improvement of industrial working conditions.
Heat, dust, and noise are all pollutants in the working environment that detract from worker safety and the stringent sanitation demands for pharmaceutical and chemical production. Tablet manufacturers can greatly reduce manual labor and diminish exposure to environmental irritants with the installation of vacuum conveying equipment that moves dry powder products through dedicated pipe systems. These systems fully contain the powders to minimize dust and also generate less heat. To ensure the highest standard of worker safety, the conveyors should also have few moving parts, and be easily assembled and disassembled to reduce worker strain.
Increasing uptime
Given the 24/7 production runs in pharmaceutical manufacturing, automation technologies must be reliable. There is no time for line stoppages or ongoing maintenance. Additionally, changeovers can add costs and downtime. A vacuum conveyor must facilitate changeover or risk negating the gains realized through automation.
?
Simple solutions can effectively combat the erosion of productivity caused by line stoppages, maintenance, or changeover. Vacuum technologies with few moving parts are not only safer; they are maintenance-free and can reduce downtime. Conveyors that are easily handled by workers are more quickly assembled and disassembled, reducing the time it takes for equipment adjustments between batches and during cleanings. Machines containing fewer components also help minimize part mix-ups and help to prevent line stoppages.
Other vacuum conveyor benefits include the following:
• Cost-effective, easy cleaning. In addition to aiming to reduce changeover time, tablet manufacturers must also prevent cross-contamination of the product. A vacuum conveyor that is easily disassembled for quick cleanup is a cost-efficient way of averting cross-contamination.
• Reduced energy consumption. As energy costs soar, the reduction of energy usage is a strategic step to trim expenses from pharmaceutical manufacturing operations. In addition, reducing energy consumption is good for sustainability efforts. Conveying systems powered by decentralized vacuum technologies are more energy-efficient than their centralized counterparts. While a centralized vacuum system puts more distance between the source of the power and the point of use, a decentralized vacuum system uses multistage ejector technology to apply the vacuum where needed. This way, no additional energy is expended to compensate for the extra distance.
• Optimized automation. Automating the tablet-handling process affords many advantages and conveniences to pharmaceutical manufacturers, including increased productivity and reduced staff injury. However, poorly designed automated conveying systems can cause segregation and tablet breakage. Simple precautions can be taken to avoid incurring the costs of product waste as a result of either event.
• Prevent segregation. Particle separation prior to tablet compression, known as segregation, can threaten the integrity of drug dosages and jeopardize the uniformity of a batch. Segregation can occur during tablet production as a result of gravity and particle characteristics, and due to external factors such as airflow and vibration. When implementing vacuum conveying, segregation risk can be reduced by handling the material at a controlled speed to ensure that the materials stay blended.
• Prevent tablet breakage. Tablets can also break due to exposure to friction and shock during or after the manufacturing process. A broken tablet is susceptible to contamination, rendering it useless. As a result, product waste can accumulate and cause production delays. To prevent breakage, tablet manufacturers can employ an accommodating vacuum conveying system. To start, the conveying system should have an adaptable feed rate to enable the system to be sped up or slowed depending on the size or volume of the tablets being handled. Also, the vacuum conveyor’s tubing and piping specifications along with couplings can be designed to reduce speed and avoid product damage. The tubing and piping should have soft curves to avoid tablet breakage.
In conclusion, vacuum automation technologies can play a key roll in streamlining tablet production. By taking the right precautions in installing a reliable, efficient vacuum conveying system, manufacturers can increase their productivity, reduce energy usage, and improve their work environments. As companies harness vacuum technology to advance tablet production, more efficient conveying systems will translate into cost savings, helping tablet manufacturers stay competitive by enabling them to produce more affordable prescriptions.
Author: Häla El Sheemy
Source: http://www.healthcare-packaging.com/archives/2009/04/improving_tablet_production_an.php
Date: 24/04/2009
Conveying of Tablets and Capsules
Overcoming the Problems of Tablet and Capsule Transfer
It is commonly acknowledged that extreme care must be taken when attempting to transport tablets and capsules from one process to another by whatever means used. The fragile nature or these products means damage can easily occur during the transport stage thus potentially wasting valuable finished goods.
Tablet and capsule handling systems must be carefully designed to avoid damage or breakages
Popular methods used for the transfer of these products are vacuum, gravity, and air all of which are standard practice within the pharmaceutical and associated industries. Hanningfield can offer much experience in this area but several points must be taken into consideration to achieve efficient material handling without damage to the finished product.
Design
Special attention has to be taken regarding design and construction materials to avoid damage to the finished product. Using specially developed design techniques it is possible to adapt standard Hanningfield process equipment to suit this highly specialised yet popular application.
For example, removable silicone liners can be supplied to fit inside vacuum transfer hoppers to avoid contact between the tablet and any metal surfaces. Specially designed air flow control valves can also be fitted to ensure when vacuum conveying of tablets, they can only move in one direction during vertical transfer cycles.
Methods
Vacuum Transfer is a well established process that can be adapted to efficiently convey coated and uncoated tablets very successfully. Internal surfaces must be specially designed to ensure the tablets do not come into contact with sharp edges or corners during transfer ('step-less conveying'). Other precautions are taken to ensure tablets will only move in one direction during transfer by use of a specially design uni-directional flow valve. The Hanningfield uni-vac system offers a number of enhanced capabilities that combine to provide safe effective tablet transfer without compromising the finished product.
Gravity is the simplest and most common transfer technique for the efficient transfer of tablets and capsules. This process is however limited to a vertical or near vertical flow path only, whereas vacuum and air powered systems can transport across horizontal distances as well as vertically upwards. To control tablet transfer, Hanningfield can supply a special flexible vane butterfly valve to minimise risk of damage when opening and closing the valve.
Air (positive pressure) is used by Hanningfield for the efficient transfer of both empty and filled hard shell capsules. Capsules are less likely to suffer from damage than tablets during conveying but internal conveying pipes must be smooth bore without ledges and sharp corners as with vacuum conveying. The positive pressure system provides a high flow, low pressure air cushion that gently moves the capsules through the convey pipe-work. Static is a common problem with capsule transfer so any system design for this application must provide an anti-static design.
Summary
In addition to good and efficient design, experience is also necessary to ensure all aspects of product protection are taken care of. This is where Hanningfield can provide the necessary input at the system concept stage to ensure a successful project conclusion using our highly skilled team of experienced engineers to guide the customer every step of the way.
Cone Mill
A cone millis an extremely effective machine for size reduction. The advantages of a cone mill over other forms of size reduction mean it is widely used in the pharmaceutical, food, chemical, cosmetic and associated industries.
So-called because of the screen shape (pictured below), a cone mill can offer great adaptability in terms of throughput, particle size, particle shape and many other factors, simply by changing the type of screen or impeller; for example, a screen with a smaller hole size will produce a smaller particle size.
Material is fed into the cone millthrough an in-feed chute. This can either be charged into the mill using a vacuum or gravity feed. The material passes to a rotating impeller which forces the material through the holes in the screen. Once the material has passed through the screen, the finished product falls from the bottom of the mill to a receptacle beneath.
The cone mill can be used for a wide variety of applications. In pharmaceuticals, they are often used for wet/dry granulation, tablet reclaim etc., in chemicals the are often used for deagglomeration, in cosmetics it can be used for fine grinding and in food it can be used for deagglomeration, product reclaim and general size reduction. For example, seeds or nuts (such as almonds, pictured below) can be ground to a smaller particle or even a powdered state.
For more information on the Hanningfield ‘Uni-Mill’ cone mill click here. Alternatively, to contact a member of our technical sales for assistance, simply click here.
Understsnding ATEX Milling
Help with Understanding ATEX Milling Offered by Hanningfield
The risk of explosion in pharmaceutical manufacturing facilities is a real possibility. To eliminate this risk, conformity and compliance with ATEX safety legislation is compulsory. Typical process areas with high risk of explosion are granulation, mixing and vacuum conveying. ATEX is the harmonised European standard created to ensure all equipment located in environments meet the specifications of the directive in terms of safety. There are currently two European directives in existence; 94/9/EC, which is principally for manufacturers and 99/92/EC, for operators of the equipment.
Previous legislation for controlling explosive atmospheres has only been concerned with electrical equipment. The current ATEX directives now include hazards from mechanical sources, such as mills. A seized bearing or mechanical failure which causes excessive heat to be generated is considered as likely to cause an explosion as an electrical fault. This is now considered to be equally important in the design and use of pharmaceutical equipment, such as mills, for powder processing.
To comply with ATEX standards a number of safety measures must be taken to eliminate any potential risk. This is initially carried out by the customer on the product to be milled via a risk analysis (Ignition Hazard Assessment), which will also evaluate the environment in which the size reduction process will take place. This assessment will determine type of hazardous zoning required inside and outside of the mill. Using the information provided, the mill supplier can then design the equipment to meet the appropriate ATEX Directive measures. The mill supplier is also responsible to advise the user of any precautions necessary to operate the mill safely.
It is imperative to match the equipment in use to the location itself when positioning equipment which is either situated in an ATEX zoned environment or contains an explosive atmosphere. If the equipment is situated in an ATEX zoned area then the external features of the mill must comply with the ATEX provisions according to the zone. Necessary precautions should be taken relating to the effect on the surrounding area and also effects resulting from the surrounding area.
With many years experience of designing and supplying explosion category milling equipment, the Hanningfield 'Uni-Mill' provides the ideal solution to meet ATEX legislation directives specifically for the pharmaceutical environment. Special features such as continuous earth design, temperature monitoring and nitrogen purging ensure appropriate compliance measures are in place for every machine supplied to meet the level of hazard specified.
Although ATEX legislation appears to be quite complex, with proper consultation between user and supplier, a safe system that meets the requirements of ATEX can be fully complied with - Hanningfield is happy to help with this.
Powder Handling Q&A
Powder Handling Q&A

As engineers look to improve their processes, one common area for improvement is powder handling. Hanningfield's Managing Director, Colin Ellis, answers some questions about powder handling and how it can help improve your process.
Q. Why is the handling of powder so important?
A. Any industry which handles powder has to consider safe and efficient movement for processing. Important factors such as reducing manual handling, maintaining cleanliness and improving containment all require careful consideration.
Powders can also contribute to health issues; this makes protection of the operators from excess dust essential to comply with health and safety legislation.
Q. What are some common methods used for powder handling?
A. There are numerous systems for the handling of powders. Amongst the most common powder handling methods are gravity, mechanical conveyor, vacuum and positive pressure. The decision must be to be based on specific application requirements, i.e. distances, material properties and available space.
Q. Are there any simple methods for handling powders?
A. The most straightforward method of powder handling is gravity. Gravity always has the least mechanical or moving parts so has to be the most cost effective and reliable. However gravity is very limited in application possibilities and material flow has to be vertical or near vertical. It is not possible to move powders over any horizontal distance using a gravity feed and obviously material can only be transported down not up.
Q. How can you effectively control the flow of powder?
A. Control of powder flow is always important to ensure feed rates or dosing amounts are as required to suit the process. Flow can be controlled manually using a simple butterfly valve or automatically using a rotary valve. Manual control tends to be intermittent whereas automatic control is generally a constant feed. More accurate dosing rates can be controlled using metering valves or screw feeders.
Q. How can you effectively contain powder?
A. Powder containment can be achieved in many ways. It is important to ensure that dust-tight interfaces are maintained between items of processing equipment. It is also necessary to consider venting closed system through a filter to displace air which would otherwise pressurise the system thus causing unnecessary powder leaks.
Ensuring at the design stage that dust tightness has fully been considered will ensure the best possible results for a contained system. Learn more about powder containment here.
Q. Are powder handling systems a good investment?
A. Return on investment is always a major consideration for capital expenditure. With powder handling there are many benefits in using an efficient system that produce immediate cost savings. Reduced manual handling and labour times can help significantly reduce production costs.
Another major benefit is faster process times, thus increasing productivity, whilst reducing both manufacturing costs and waste material. Less spillage not only saves on waste cost but also creates a cleaner safer workplace reducing cleaning time and minimising the possibility of accidents.
To learn more about various powder handling solutions, please click HERE.
Do you need more "Tips & Hints' for you process applications?
Just fill in the form on this page and receive our regular FREE "Process Tips & Hints" Newsletter right into your mailbox.


















 Unknown
Unknown Unknown
Unknown Mozilla
Mozilla